Acidic (low pH) Drinking Water
- Acidic (low pH) Drinking Water
- Arsenic in Well Water
- Barium In Well Water
- Cadmium in Well Water
- Chloride in Drinking Water
- Chromium in Drinking Water
- Coliform Bacteria in Drinking Water
- Color in Drinking Water
- Copper in Drinking Water
- Cyanide In Well Water
- Environment Guide
- Fluoride in Drinking Water
- Iron in Drinking Water
- Lead in Drinking Water
- Mercury in Well Water
- Nitrates in Well Water
- Nitrite in Drinking Water
- Odors in Drinking Water
- Pesticides in Drinking Water
- Radium in Well Water
- Selenium in Drinking Water
- The Effects of Water Pollution
- Turbidity and Cloudiness of Drinking Water
- Viruses in Drinking Water
- Water Contaminants
- Water Pollution
- Well and Ground Water Contamination
Important Topics

Acids are known for their corrosive properties, and even mildly acidic drinking water can corrode pipes, leading to contamination of the water with harmful chemicals.
Acidic water can result from a number of natural causes. Most rainwater is mildly acidic, and plant roots and microbes in soil produce carbon dioxide and other acids. Many sediments and sedimentary rocks contain enough acid-neutralizing minerals to neutralize most of this acidity, but most igneous and metamorphic rocks have a poor capacity to neutralize acid, resulting in acidic shallow groundwater. This problem can be made worse when acid rain (rain that is more than mildly acidic) occurs. River and lake water can also be acidic.
“50% or more of drinking water wells are acidic in some states.”
Acidic groundwater can also result from interaction with acid-producing minerals or contact with organic matter. These processes can affect both shallow and deep groundwater. It is very common for groundwater to be acidic, as 50% or more of drinking water wells are acidic in some states. These types of chemical processes can lower the pH of the water, leading to problems with water quality.
What is pH?
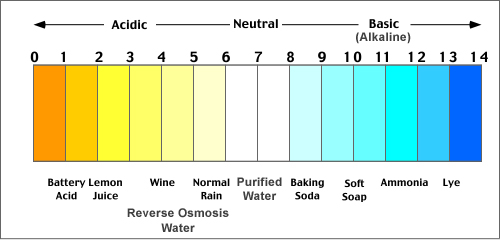
pH is a measure of how acidic or basic water is. The pH scale is arranged so that a value of 7 represents a neutral solution, values less than 7 represent acidic water, and values greater than 7 represent basic (alkaline) water. The pH scale is often incorrectly stated as ranging from 0 to 14, but there are actually no definite upper or lower limits to the scale – negative values of pH are even possible! A pH of 3 in well water would be highly acidic, whereas values ranging from 5 to 6.5 are mildly acidic.
pH values of water can be tested using inexpensive home test kits.
Interesting fact: Water with a pH as low as -3.6 was observed in a mine in California.
What is Acidity?
Acidity is probably the most widely misunderstood concept in water chemistry. Acidity is a chemical quantity that represents the amount of base that is required to neutralize all of the acids in the water. One would generally expect water with a lower pH (water that is more acidic) to have a higher value of acidity, but this is not always the case. The relationship between pH and acidity actually depends on the types and amounts of acids present in the water. Water that is less acidic can have a higher value of acidity, and water that is alkaline can even have a positive value of acidity. No wonder people can get confused!
Acidity is often reported in terms of milligrams of calcium carbonate per liter (mg CaCO3 / L). Conveniently, this indicates the amount of calcium carbonate (lime) needed to neutralize all of the acids in the water. Measurement of acidity is best performed by a lab.
Corrosion of Pipes
The biggest problem with acidic drinking water is that acidic water tends to be corrosive to pipes. Not only can this damage pipes, it can also result in contamination of your water with lead (from lead-containing solder and fixtures), copper (from copper pipes), and other heavy metals (such as iron, manganese and zinc), as well as vinyl chloride from low-quality plastic pipes. Some signs of corroding pipes include bluish-green stains in sinks (if you have copper pipes), reddish stains (if you have galvanized iron pipes), a bitter metallic taste to the water, and the development of small leaks in pipes.
If you suspect you have a lead problem , start by testing your water for lead, or more comprehensive water test. If lead is coming from your well or municipally supplied water, use one of the lead removing filters that we offer. Read below about how to neutralize acidic water (pH below 6.5).
Health Effects Associated with Low-pH (acidic) Water
“The presence of acidic water can lead to unacceptable contamination of the water supply by lead and other toxic heavy metals.”
Although acidic water by itself is generally not a health concern, the presence of acidic water can lead to unacceptable contamination of the water supply by lead and other toxic heavy metals. The U.S. Environmental Protection Agency (USEPA) has set a secondary maximum contaminant level (SMCL) for pH of 6.5 to 8.5, meaning that the pH of municipal water supplies should be between these two values. Municipal water supplies are not required to meet this standard, but this serves as a guide for what the target pH of your water supply should be.
How to Raise the pH (or Neutralize the Acidity) of Drinking Water
The pH of water can be lowered by neutralizing filters or chemical feed systems. Because the entire plumbing system should be protected from the corrosive water, the water should be treated in a point-of-entry (POE) system where it first enters the building.
Acid-neutralizing filters work by dissolving material such as calcium carbonate (lime) to neutralize the acid. The unit consists of a tank filled with chips of limestone, marble or other alkaline material. This consequently increases the hardness of the water, so further treatment of the water for hardness by water softening may be desired. The tank must be routinely refilled as the material dissolves.
Chemical feed systems work by adding acid-neutralizing chemicals such as sodium hydroxide (lye) or soda ash to the water through a chemical feed pump. Soda ash is considered to be preferable for home systems because it is safer to handle. This type of system does not increase the hardness of the water. Periodic maintenance, such as filling the solution tanks and maintaining the feed pump, is required.

 View Cart
View Cart